|
|
|
| AIDScience Vol. 3, No. 21, 2003 |
| Calculating the potential epidemic-level impact of therapeutic vaccination on the San Francisco HIV epidemic |
| Sally Blower,1 Ronald B. Moss,2 Eduardo Fernandez-Cruz3 |
| 1Department of Biomathematics, David Geffen School of Medicine, University of California at Los Angeles, California, United States 2The Immune Response Corporation, Carlsbad, California, United States 3Comunidad de Madrid, Hospital General Universitario Gregorio Marañón, Madrid, Spain |
| Address correspondence to: sblower@mednet.ucla.edu |
| ||||||||||||||||||
Abstract
![]() he goal of the current study was to predict the potential epidemic-level impact of an HIV-1 therapeutic vaccine when combined with antiretrovirals (ARV), in the San Francisco gay community. We designed a mathematical model and calculated the potential effectiveness of two types of vaccines in terms of (i) the number of HIV infections prevented per year and (ii) the number of AIDS deaths prevented per year. We modeled two types of vaccine: (i) a vaccine that increased survival slightly but had no effect on transmission, and (ii) a vaccine that increased survival slightly and also reduced transmission. We parameterized our model to reflect the HIV epidemic in the gay community in San Francisco, where HIV prevalence is 30%, and usage of ARV is very high. We used parameter values for the therapeutic vaccines based upon a recently reported clinical trial (STIR 2102); hence, we assumed that the vaccines would add (on average) one additional year to life expectancy, and that in addition one of the vaccines could reduce infectiousness by 37%. Our calculations showed that over a 20 year time period the therapeutic vaccine that only increased survival had an almost negligible impact on the epidemic. However, the therapeutic vaccine that increased survival and also decreased infectiousness had a substantial impact on the epidemic: over a 20 year period this vaccine would prevent 26%-27% of HIV infections, and 9-10% of AIDS deaths in San Francisco. Our results suggest that therapeutic vaccination used in combination with ARV could be useful in decreasing the HIV epidemic.
he goal of the current study was to predict the potential epidemic-level impact of an HIV-1 therapeutic vaccine when combined with antiretrovirals (ARV), in the San Francisco gay community. We designed a mathematical model and calculated the potential effectiveness of two types of vaccines in terms of (i) the number of HIV infections prevented per year and (ii) the number of AIDS deaths prevented per year. We modeled two types of vaccine: (i) a vaccine that increased survival slightly but had no effect on transmission, and (ii) a vaccine that increased survival slightly and also reduced transmission. We parameterized our model to reflect the HIV epidemic in the gay community in San Francisco, where HIV prevalence is 30%, and usage of ARV is very high. We used parameter values for the therapeutic vaccines based upon a recently reported clinical trial (STIR 2102); hence, we assumed that the vaccines would add (on average) one additional year to life expectancy, and that in addition one of the vaccines could reduce infectiousness by 37%. Our calculations showed that over a 20 year time period the therapeutic vaccine that only increased survival had an almost negligible impact on the epidemic. However, the therapeutic vaccine that increased survival and also decreased infectiousness had a substantial impact on the epidemic: over a 20 year period this vaccine would prevent 26%-27% of HIV infections, and 9-10% of AIDS deaths in San Francisco. Our results suggest that therapeutic vaccination used in combination with ARV could be useful in decreasing the HIV epidemic.
The level of plasma HIV-1 RNA has been shown to predict the rate of clinical disease progression in HIV-1 infected individuals (1, 2). Furthermore, the level of HIV-1 RNA predicts the probability of transmission (3). Therefore, therapies that decrease HIV-1 RNA can potentially benefit both individual patients as well as reduce epidemic severity. Mathematical modeling analyses have shown that combination antiretroviral (ARV) therapies can significantly reduce the number of new infections, and the AIDS death rate (4-6). Mathematical modeling has also been coupled with cost-effectiveness analysis to estimate the effectiveness and efficiency of U.S. HIV prevention efforts (7). Here, we developed a new mathematical model to examine the potential epidemic-level impact of the addition of an HIV-1 therapeutic vaccine to ARV therapies. Specifically, we calculated the potential epidemic-level impact of a therapeutic vaccine when used with ARV in terms of (i) the number of HIV infections that could be prevented per year, and (ii) the number of AIDS deaths that could be prevented per year in the gay community in San Francisco.
In order to make these calculations we developed a new mathematical model consisting of six ordinary differential equations. The model allows HIV-infected individuals to receive ARV at any time, but they would only be eligible to receive the therapeutic vaccine in combination with ARV in the first stage of infection. We defined this vaccination eligibility period to last from 0 to 5 years post-infection. We modeled the effects of ARV and the therapeutic vaccine in terms of both increasing survival and reducing the probability of transmission (by reducing infectiousness). We parameterized our model to reflect the HIV epidemic in the gay community in San Francisco, where HIV prevalence is currently 30%. We assumed a high ARV usage rate of 90%. We modeled two types of vaccine: (i) a vaccine that increased survival slightly but had no effect on transmission, and (ii) a vaccine that increased survival slightly and also reduced infectiousness. We used parameter values for the vaccines that reflected the results from a recently reported clinical trial STIR 2102 of a therapeutic vaccine used in combination with ARV (8). In this trial the therapeutic vaccine Remune appeared to add one additional year to survival, and decreased viral load by 37% (8).
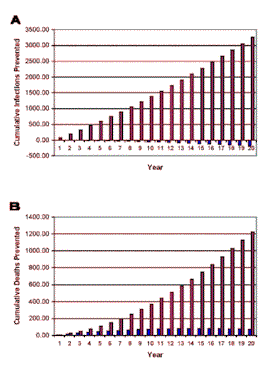 |
| Figure 1. [Enlarge] Predicted potential epidemic-level impact of an HIV-1 therapeutic vaccine, when combined with a 90% rate of ARV therapy, on the HIV epidemic in the gay community in San Francisco. A mathematical model (see Supporting Online Material) was used to predict the effect of the therapeutic vaccine over time on (A) the cumulative number of HIV infections prevented, and (B) the cumulative number of AIDS deaths prevented. The epidemic-level effects of two types of vaccine are shown: (i) a vaccine that increased survival by one year but had no effect on transmission (data in blue), and (ii) a vaccine that increased survival by one year and also reduced transmission (data in red). We assumed that patients treated only with ARV would survive on average 15 years; all other parameter values are given in the text. |
The model tracked the transmission dynamics of HIV in the presence of ARV and a therapeutic vaccine. We used the model to calculate the number of AIDS deaths prevented per year and the number of HIV infections prevented per year. The model consisted of 6 ordinary differential equations (see Supporting Online Material). The potential treatment strategies were stratified on the time since infection: HIV-infected individuals could receive ARV at any time, early or late stage of infection, but they could only receive the therapeutic vaccine in combination with ARV in the first stage of infection, set to be 5 years post-infection in the current analysis but which could be of any length. Thus, two treatment strategies were modeled: ARV alone or ARV plus the therapeutic vaccine. The model allowed ARV and the therapeutic vaccine to independently alter both the disease progression rate and the probability of treated HIV-infected individuals transmitting HIV by reducing their viral load.
The model tracked the temporal dynamics of the number of individuals in each of six states that are specified by the six state variables: the number of susceptible uninfected individuals (X), the number of untreated individuals in the early stage of HIV-infection (YEU), the number of treated (with ARV only) individuals in the early stage of HIV-infection (YEA), the number of treated (with ARV and therapeutic vaccine) individuals in the early stage of HIV-infection (YEAV), the number of untreated individuals in late stage of HIV-infection (YLU), and the number of treated (with ARV only) individuals in late stage of HIV-infection (YLA). Individuals in the five different HIV-infection states can progress to AIDS at different rates and transmit HIV with different probabilities, due to differences in their viral load (See Supporting Online Material for further details).
We parameterized the model to reflect the transmission dynamics of HIV in the gay community in San Francisco (4), which has a 30% prevalence of HIV infection. To reflect the situation in that city we specified high treatment rates: the fraction of early HIV infections treated only with ARV (FEA) to be 0.45 (i.e., 45%), the fraction of early HIV infections treated with ARV plus vaccine (FEAV) to be 0.45 (i.e., 45%) and the fraction of late HIV infections treated only with ARV (FLA) to be 0.8 (80%). We assumed that 15-18% of treated patients would give up treatment per year; hence, we set gEA = 0.15 per year, gEAV = 0.18 per year, and gLA = 0.15 per year. We set the early stage of infection when individuals would be eligible for the therapeutic vaccine to be 5 years (hence 1/vEU = 5 years and 1/vLU = 5 years), the average survival time if on ARV only and received treatment early (1/vEA) to be 15-20 years, the average survival time if on ARV and the therapeutic vaccine and received treatment early (1/vEAV) to be 16-21 years, and the average survival time if on ARV only and received treatment late (1/vLA) to be 10 years. We assumed that the transmission probability of untreated individuals would be 0.1 (hence, βEU = βLU = 0.1), that ARV would reduce transmissibility by 50% (hence βEA = βLA = 0.05), and that the vaccine, if it reduced infectiousness by reducing viral load, would reduce transmissibility by an additional 37% (hence, βEAV = 0.013).
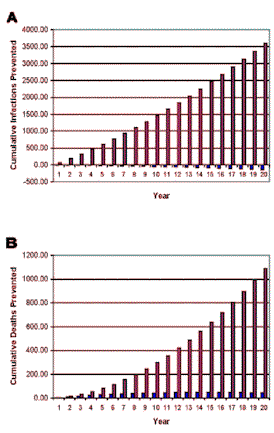 |
| Figure 2. [Enlarge] Predicted potential epidemic-level impact of an HIV-1 therapeutic vaccine, when combined with a 90% rate of ARV therapy, on the HIV epidemic in the gay community in San Francisco. A mathematical model (see Supporting Online Material) was used to predict the effect of the therapeutic vaccine over time on (A) the cumulative number of HIV infections prevented, and (B) the cumulative number of AIDS deaths prevented. The epidemic-level effects of two types of vaccine are shown: (i) a vaccine that increased survival by one year but had no effect on transmission (data in blue), and (ii) a vaccine that increased survival by one year and also reduced transmission (data in red). Parameter values are all the same as used to generate Figure 1 except we assumed that patients treated only with ARV would survive on average 20 years. |
We modeled the potential effect that a therapeutic vaccine used in combination with ARV would have on the HIV epidemic in the gay community in San Francisco. We modeled two types of therapeutic vaccine: (i) a vaccine that increased survival by one year but had no effect on transmission, and (ii) a vaccine that increased survival by one year and also reduced transmission. For each of the two types of vaccines, we compared their potential effects with two baseline simulations, where we assumed that only ARV was available and treated individuals average survival time was either 15 years or 20 years. We predicted the epidemic-level effects of these therapeutic vaccines for a 20 year period.
Our simulations showed that if only ARV was used in the gay community, over a 20 year time period 12,559-13,441 cumulative new infections and 10,915-11,845 cumulative AIDS deaths would occur. We calculated the effects of the two types of therapeutic vaccines when used in addition to ARV in terms of (i) the cumulative number of additional HIV infections prevented per year, and (ii) the cumulative number of additional AIDS deaths prevented per year. Results are shown in Figure 1, assuming individuals treated with ARV live an average of 15 years, and Figure 2, assuming individuals treated with ARV live an average of 20 years. The data in blue show the impact of the therapeutic vaccine that only increases survival by one year, and the data in red show the impact of the therapeutic vaccine that increases survival by one year and also decreases transmission.
Over a 20 year time period it can be seen that the therapeutic vaccine that would only increases survival by one additional year had an almost negligible epidemic-level impact: only a few (44-75) additional AIDS deaths were prevented (Figure 1B and 2B; data in blue), and the number of new HIV infections slightly (137-178) increased over this time period (Figure 1A and 2A; data in blue). However, the therapeutic vaccine that would increase survival by one additional year and also would decrease transmission could have a substantial epidemic-level impact: over a 20 year period this vaccine would prevent 3,261-3,599 HIV infections (Figures 1A and 2A; data in red), and 1,091-1,229 AIDS deaths (Figure 1B and 2B; data in red). Therefore this type of therapeutic vaccine would prevent 26%-27% of HIV infections, and 9%-10% of AIDS deaths over a 20 year period.
Moderately effective imperfect HIV vaccines could substantially reduce the HIV epidemic, particularly if coupled with changes in risk behavior (9-11). Here, we have calculated the potential effects at the epidemic-level of modestly effective therapeutic vaccines. Our calculations showed that if a therapeutic vaccine used together with ARV prolonged survival and also reduced infectiousness, if used at high coverage levels, it could potentially have a fairly significant impact at the population level. We have specifically evaluated the potential impact of such a therapeutic vaccine if used with high levels of ARV in the gay community in San Francisco. It is possible that such therapeutic vaccines could also be beneficial in other communities in the developed and developing world, as access to ARV increases. Our model could be used to investigate this possibility, and hence the applicability of our results for other communities. However, our current results suggests that safe and modestly effective immune-based therapies that work in combination with ARV could potentially result in substantial public health benefits in San Francisco.
References and notes
| 1. | J.W. Mellors, et al., Ann. Intern. Med. 126, 946 (1997). PubMed |
| 2. | W.A. O'Brien, P.M. Hartigan, E.S. Daar, M.S. Simberkoff, J.D. Hamilton, Ann. Intern. Med. 126, 939 (1997). PubMed |
| 3. | T.C. Quinn, et al., N. Engl. J. Med. 342, 921 (2000). PubMed |
| 4. | S.M. Blower, H. Gershengorn, R. Grant, Science 287, 650 (2000). PubMed |
| 5. | S.M. Blower, A.N. Aschenbach, H.B. Gershengorn, J.O. Kahn, Nat. Med. 7, 1016 (2001). PubMed |
| 6. | J.X. Velasco-Hernandez, H.B. Gershengorn, S.M. Blower, Lancet Infect. Dis. 2, 487 (2002). PubMed |
| 7. | D.R. Holtgrave, AIDS 16, 2347 (2002). PubMed |
| 8. | E. Fernandez-Cruz, et al., paper presented at the 10th Conference on Retroviruses and Opportunistic Infections, Boston, MA, February 2003. Available online |
| 9. | S. Blower, A.R. McLean, Science 265, 1451 (1994). PubMed |
| 10. | S. Blower, K. Koelle, D.E. Kirschner, J. Mills, Proc. Natl. Acad. Sci. USA 98, 3618 (2001). PubMed |
| 11. | S. Blower, E.J. Schwartz, J. Mills, AIDS Rev. 5, 113 (2003). PubMed |
| 12. | This work was funded by The Immune Response Corporation. |
| Copyright Information | Site map |