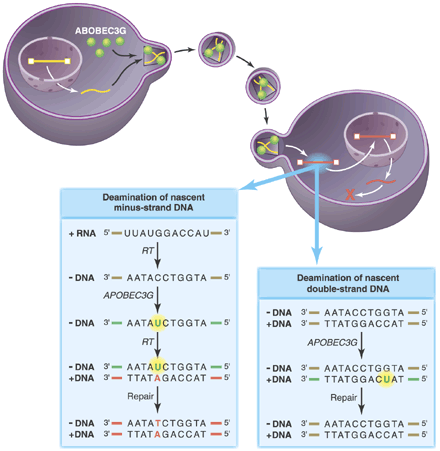
APOBEC3G on the warpath. Retrovirus production in a nonpermissive cell expressing APOBEC3G results in the incorporation of APOBEC3G (green) into viral progeny. (Left box) Although these virions are able to invade host cells and undergo the early steps of reverse transcription, the APOBEC3G cytidine deaminase induces rapid accumulation of deoxyuridine residues (green) at mutagenic hotspots in the nascent minus strand of the transcribed retroviral DNA. These mutations diminish second-strand DNA synthesis by reverse transcriptase, increasing the lability of the viral genome. Copying of the mutated base into plus-strand DNA and repair by cellular systems makes the mutation permanent (red) and integration of the viral DNA genome proceeds, resulting in a hypermutant provirus. (Right box) Mutations introduced into double-stranded DNA are likely to be repaired correctly. The presence of the viral protein Vif in the infected host cell prevents the incorporation of APOBEC3G into retroviral particles.
CREDIT: KATHARINE SUTLFIFF/SCIENCE