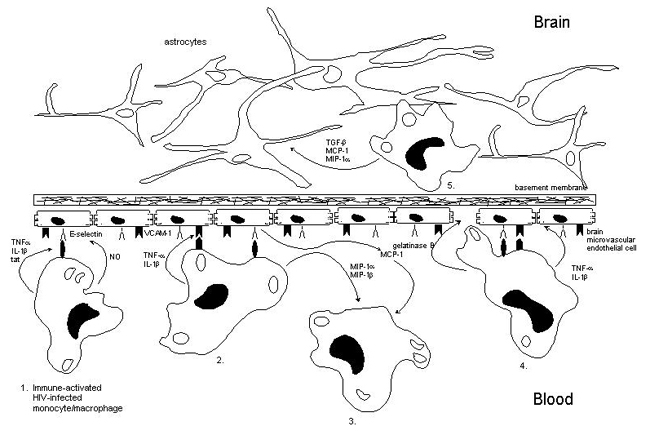
(1) During the later symptomatic stages of HIV-1 infection elevated levels of pro-inflammatory cytokines such as TNF-a and IL-1b are secreted by perivascular HIV-1-infected M/M. TNF-a, IL-1b and HIV-1 tat induce the expression of E-selectin on BMEC. The change that these cells indeed roll on brain endothelium via E-selectin might increase due to the secretion of NO, a potent vasodilator, by HIV-infected macrophages. E-selectin then mediates rolling of HIV-infected M/M on the vessel wall.
(2) TNF-a and IL-1b also induce the expression of VCAM-1 on BMEC. The induction of this adhesion molecule allows binding of HIV-infected M/M to brain endothelium.
(3) The interactions between HIV-infected M/M and BMEC result in the production of the M/M-derived chemokines MIP-1a, MIP-1b and the endothelial-derived chemokine MCP-1. These chemokines will attract more mononuclear phagocytic cells into the CNS.
(4) The overexpression of the pro-inflammatory mediators TNF-a, and IL-1b by HIV-infected M/M will result in an increase in blood-brain barrier (BBB) permeability. The overexpression of gelatinase B by HIV-infected M/M will result in degradation of abluminal extracellular matrix proteins. All these factors will lead to the transmigration of HIV-infected M/M across the BBB.
(5) Intercellular interactions between astrocytes and infiltrated HIV-infected M/M within the brain parenchyma will result in the induction of several chemokines, TGF-b, MIP-1a and MCP-1. These chemokines will attract more HIV-infected and uninfected M/M into the brain parenchyma resulting in an expansion of the viral load within the CNS.