
ASIA--THE NEXT FRONTIER FOR HIV/AIDS:
Thailand Beats the Odds in Completing Vaccine Test
Jon Cohen
A just-completed efficacy trial in injecting drug users is a major accomplishment. Even the company behind the trial wondered if it could be done
BANGKOK, THAILAND--Gulping down a tin cup full of yellow syrup, a 32-year-old man visiting this clinic at Taksin Hospital for his daily dose of methadone explains that after 14 years of injecting heroin, he recently became infected with HIV. "I had a wife, but she left me when I told her about my blood results," says the man, who ekes out an existence as a day laborer and lives with his father. "I knew that HIV was transmitted by sharing needles, but I had a craving."
His tragic circumstance is, unfortunately, not unusual: A catastrophic 40% of injecting drug users (IDUs) in Thailand are infected with HIV. But his infection is particularly noteworthy because it occurred while he was participating in a landmark AIDS vaccine study, just now coming to a close. In the painful calculus of a placebo-controlled vaccine efficacy trial, some people must become infected for researchers to determine whether a preparation has any worth. (Until the data are unblinded, it will not be known whether he was given a placebo or the vaccine.)
Regardless of the ultimate results, just completing the study--the first AIDS vaccine efficacy trial ever held in a developing country--marks a significant achievement. "It took one-and-a-half years for us to get this study past six committees," says the project's director, Kachit Choopanya, a pediatrician who shifted into treating IDUs in the 1970s. "This has been very hard for us." The researchers screened 4944 IDUs who volunteered for the study, a collaboration between the Thai Ministry of Public Health, the U.S. Centers for Disease Control and Prevention, the Bangkok Metropolitan Administration, and the vaccine's manufacturer, VaxGen of Brisbane, California. From that initial group, the researchers selected 2545 uninfected people to receive either a placebo or the vaccine, a genetically engineered version of HIV's surface protein, gp120, made from HIV strains circulating in Thailand. By the time the study formally came to a close this June, the 380 staff members working in Taksin and the other 16 clinics involved in the study had given more than 17,000 injections, drawn more than 40,000 blood samples, and processed a half-million forms charting the results.

Thai PI. Kachit Choopanya ran the 2500-person study.
PHOTO BY MALCOLM LINTON
VaxGen co-founder Donald Francis says that when a colleague first suggested conducting this efficacy study in Thai IDUs, he was highly skeptical. "I said, 'Oh my god, we can't follow them for anything.' " Yet fully 95.6% of the participants showed up for all three injections given during the first 6 months, and by mid-May 2003, 84.4% had received four more booster shots. Francis attributes the high retention rate to the deep trust that the IDU community has for Kachit, mixed with pride that participants felt in joining the study. "There aren't many opportunities for drug users to feel that way," he says.
The high retention rate is especially remarkable because at each study visit, 30% of the participants reported having spent time in jail or prison during the preceding 6 months. "We didn't really expect that when we started," says Francis. The researchers conducted more than 2000 visits to incarcerated trial participants, collecting blood samples and giving the vaccine.
Before they began the trial, the researchers had to confront a sticky ethical question: Could participants be placed at greater risk of becoming infected? The concern does not center on the vaccine itself, which contains a harmless piece of HIV that cannot cause an infection, but on the possibility that participants might indulge in riskier behavior if they assumed that they had received the vaccine and that it offered some protection. However, a 1-year analysis of participants--who all received risk- reduction counseling at each study visit--showed that injecting drug use declined from 93.8% to 66.6%, and needle sharing dropped from 33% to 17.5%.
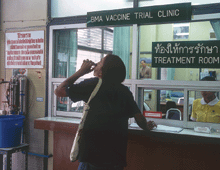
Strong medicine? Data from this methadone clinic at Taksin Hospital will help assess whether the vaccine works.
PHOTO BY MALCOLM LINTON
The decision made by the 32-year-old man at Taksin Hospital to share a needle pinpoints one reason why someone who understood this risk would take it. "We didn't have enough money to buy heroin for each one of us, so we pooled our bhat," he explains, describing an incident with friends that he thinks led to his infection. "We shared the same syringe so that each one of us received our fair share." A syringe provides an ideal calibration device, as it has lines that precisely demarcate one cubic centimeter of liquefied heroin from another. Risk-reduction strategies typically ignore this vexing reality.
The much-anticipated results from the study are likely to receive exceptional scrutiny. VaxGen sparked a red-hot controversy last February when it announced the results of a separate efficacy study conducted in North America, the Netherlands, and Puerto Rico. That trial, which mainly enrolled gay men at high risk of sexual transmission, found that the vaccine offered no more protection from HIV infection than did the placebo. But the company claimed that the vaccine inexplicably seemed to work remarkably well in a small subset of black participants and possibly Asians, too--a subset analysis that came under harsh criticism (Science, 7 March, p. 1495).
Although researchers roundly dismissed the notion that race would affect how well a vaccine worked, many remain open to the idea that the different route of transmission in this trial could lead to a different outcome. The Thai study group plans to reveal its results before the end of the year.
Volume 301,
Number 5640,
Issue of 19 Sep 2003,
p. 1663.
Copyright © 2003 by The American Association for the Advancement of Science. All rights reserved.
|