|
|
|
| AIDScience Vol. 1, No. 12, October 2001 |
Anti-HIV-1 microbicide potential of the tight-binding nonnucleoside reverse transcriptase inhibitor UC781
Gadi Borkow,*1 and Michael A. Parniak2
1R. Ben-Ari Institute of Clinical Immunology, AIDS Center, Kaplan Medical Center, Rehovot 76100, Israel
2Department of Medicine, Division of Infectious Diseases, Scaife Hall, University of Pittsburgh, Pittsburgh, Pennsylvania 15261, United States
*To whom correspondence should be addressed. E-mail: gadi_borkow@clalit.org.il
Abstract
| Related Links | |
| • | dEbates: Submit a response to this article |
| • | Reprint
(PDF) version of this article (126 KB)  |
| • | [Get PDF Reader] |
![]() he main mode of HIV-1 transmission worldwide is through heterosexual intercourse. Microbicides, i.e., female-controlled, intravaginal HIV-1 neutralizing formulations, may provide a means to reduce the spread of the virus. A variety of compounds and formulations have been proposed as anti-HIV microbicides, including UC781, a tight-binding nonnucleoside reverse transcriptase (RT) inhibitor. UC781 has significant potential for anti-HIV microbicide use, in that short pretreatment both of isolated cells and human cervical tissue explants with low concentrations of UC781 provides a strong barrier to subsequent virus infection, whether by cell-free or cell-associated HIV-1. UC781 is readily formulated into carriers appropriate for vaginal application, such as ReplensTM gel, and has a favorable toxicity profile. These factors suggest that UC781 warrants further clinical assessment for use in topical anti-HIV microbicide applications.
he main mode of HIV-1 transmission worldwide is through heterosexual intercourse. Microbicides, i.e., female-controlled, intravaginal HIV-1 neutralizing formulations, may provide a means to reduce the spread of the virus. A variety of compounds and formulations have been proposed as anti-HIV microbicides, including UC781, a tight-binding nonnucleoside reverse transcriptase (RT) inhibitor. UC781 has significant potential for anti-HIV microbicide use, in that short pretreatment both of isolated cells and human cervical tissue explants with low concentrations of UC781 provides a strong barrier to subsequent virus infection, whether by cell-free or cell-associated HIV-1. UC781 is readily formulated into carriers appropriate for vaginal application, such as ReplensTM gel, and has a favorable toxicity profile. These factors suggest that UC781 warrants further clinical assessment for use in topical anti-HIV microbicide applications.
Introduction
According to the Joint United Nations Program on HIV/AIDS (UNAIDS), in the 20 years since AIDS was first identified, almost 19 million people have died from this disease. Furthermore, there are more than 33 million people presently infected by HIV-1; almost half of these are women (1). HIV/AIDS is therefore an epidemic of staggering proportions. Despite intensive effort, no effective anti-HIV vaccine is yet available. Although prophylactic vaccination is, and must remain, a key objective in the fight against AIDS, other means should also be considered to minimize the spread of HIV.
The main mode of HIV-1 transmission worldwide is through heterosexual vaginal intercourse (2). Although the use of male condoms is quite effective in preventing transmission the agents responsible for sexually transmitted diseases (STDs), including HIV, condom breakage and slippage during intercourse may occur (3, 4). Furthermore, male condom use is socially or culturally unacceptable or both in certain populations, including some where HIV infection is rampant (5, 6). Thus, there is an increasing awareness that strategies to prevent heterosexual transmission of STDs including HIV must include female-controlled methods. This is especially important in areas of where the prevalence of HIV is high and for women with multiple sexual partners, factors that are associated with higher risk for both acquisition and transmission of HIV.
Topical anti-HIV microbicides, i.e., female-controlled, intravaginal formulations that can prevent HIV-1 transmission, represent one such strategy. Table 1 summarizes several requirements that an ideal topical anti-HIV microbicide should fulfill. Anti-HIV vaginal microbicide formulations need not also possess spermicidal activity, although such spermicidal utility may help to promote microbicide use in some cases. There is also some debate as to whether topical anti-HIV microbicides should be specific for HIV or should be developed to also provide broad-spectrum protection against a range of STD-causing agents. The severity of the ever-increasing HIV pandemic suggests to us that efforts should be concentrated on maximizing efficacy of microbicides specifically targeting HIV transmission.
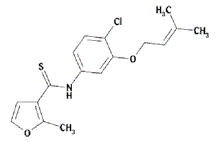 |
|
| Figure 1. Structure of UC781. | |
| [View larger version of this image] | |
A variety of agents have been proposed as topical anti-HIV microbicides (see 7-9 for reviews). These include surfactants that disrupt the virus membrane integrity, such as nonoxynol-9. Unfortunately, nonoxynol-9 has failed in human clinical trials, due to the drug-induced formation of localized genital lesions that might in fact actually promote virus transmission. Other potential microbicides include inhibitors of virus adsorption, such as Dextrin sulfate or PRO 2000, and viral fusion inhibitors. Finally, antivirals that inhibit a key function of HIV replication prior to integration, such as reverse transcriptase (RT) inhibitors, may be useful in microbicide applications. The nucleotide inhibitor PMPA (Tenofovir) has been suggested for this purpose (9), but PMPA requires cellular activation to the diphosphate for antiviral activity, and is therefore unlikely to act directly to inactivate HIV virions. Nonnucleoside reverse transcriptase inhibitors (NNRTIs) have also been suggested as potential microbicides (7). However, our work has shown that not all NNRTIs have anti-HIV microbicidal activity (10, 11). NNRTIs are rather lipophilic compounds, and therefore should readily enter membrane milieux such as the cell plasma membrane or the membrane envelope surrounding the HIV core. Although this characteristic is a prerequisite for the compound to find and interact with HIV-1 virion RT, this property would also facilitate efflux of the compound from the virion, in the absence of extra-virion inhibitor. In order for NNRTIs to be effective virucides (in other words, to "irreversibly" inactivate the virion), they should possess some other property or properties that will prolong the inhibitor residence within the virion.
One NNRTI with demonstrated potential for use in topical anti-HIV microbicide formulations is the thiocarboxanilide UC781. This review describes the current status of the development of this compound for microbicidal applications.
Antiviral properties of UC781
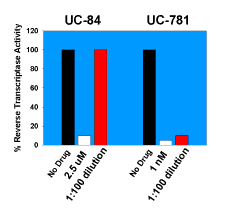 |
| Figure 2. An illustrative experiment showing that UC781 is a tight-binding inhibitor of HIV-1 RT. HIV-1 RT was incubated with 2.5 µM UC84 or with 1 nM UC781 for 10 minutes at 4°C. Excess unbound NNRTIs were removed by "spin-column" gel chromatography. Although almost complete recovery of RT activity was noted with the enzyme treated with UC84, virtually no recovery of the RT treated with UC781 was noted following removal of excess UC781. Detailed kinetic studies describing the rapid tight-binding inhibition of HIV-1 RT by UC781 are described in 20. |
| [View larger version of this image] |
UC781 is one of a large series of carboxanilide derivative NNRTIs, developed by Uniroyal Chemical Inc. (now Crompton, Inc.). The first series of carboxanilides was designed and synthesized as analogs of the commercial fungicide VitavaxTM. One of these (UC84) was identified in the National Cancer Institute AIDS drug-screening program as possessing reasonably potent anti-HIV-1 activity (12). Based on these initial findings, Uniroyal chemists prepared more than 1400 structurally related compounds, resulting in compounds with significantly improved antiviral activity against both laboratory and clinical strains of HIV-1, including both syncytium and non-syncytium-inducing phenotypes, and with excellent potency against several NNRTI-resistant strains of HIV-1 (13-19). This development has culminated in UC781, one of the most potent NNRTIs so far described (Fig. 1).
Microbicidal properties of UC781
In addition to its excellent antiviral activity, UC781 possesses distinctive characteristics that affect its utility as an anti-HIV-1 microbicide, namely, that it is a tight-binding inhibitor of HIV-1 RT (20), the first NNRTI to be identified with this property (Fig. 2). Tight-binding inhibitors are those that bind rapidly to the target enzyme, but once bound, dissociate very slowly, on the order of minutes to hours. In addition, tight-binding inhibitors may show potent inhibition at stoichiometric (1:1) ratios with the target enzyme; UC781 has this property. The tight-binding nature of UC781 inhibition suggested to us that if the lipophilic UC781 could penetrate the HIV-1 membrane envelope and capsid cores and bind to intravirion RT, the drug would not readily dissociate from the enzyme and would therefore be "trapped" within the virion, even when extra-virion drug is removed. This would have the net effect of "inactivating" the HIV virion.
We found that short exposure of isolated HIV-1 virions to submicromolar concentrations of UC781 completely inactivated the virus and that this inactivation persisted even following removal of extra-virion drug (10). Nevirapine and UC84, neither of which are tight-binding inhibitors of RT, were able to inactivate HIV in this manner (Fig. 3). The short exposure of the virions to UC781 also dramatically inhibited endogenous reverse transcription in these virions (10). Endogenous reverse transcription has been implicated as an important factor in sexual transmission of HIV-1 infection (21, 22).
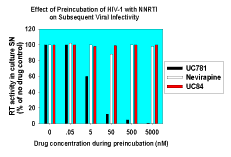 |
| Figure 3. UC781 inactivates isolated HIV-1 virions. Isolated HIV-1 was incubated with UC781, UC84, or nevirapine for 2 hours at 37°C. Residual drug was removed by centrifugal concentrators. Identical viral inocula were then added to phytohemagglutinin-activated cord blood mononuclear cells. Viral infectivity was assessed by measurement of RT activity in the cell-free supernatants after 7 days of incubation. A residual drug effect control, in which 500 nM UC781 was carried through the dilution and concentration steps as for pretreated virus and then added to culture supernatants, revealed no effect on viral infectivity and on RT activity in the in vitro assay. |
| [View larger version of this image] |
UC781 also abrogates the infectivity of cell-associated virus. Exposure to UC781 of peripheral blood lymphocytes from HIV-infected individuals prevented these cells from subsequently transmitting HIV to uninfected cells in the absence of exogenous drug (10). Similar results were noted with chronically-infected H9 lymphoblastoid cells treated with UC781. Although this treatment had no effect on the production of virus following removal of the exogenous drug, these nascent virions were non-infectious. This suggests that UC781 may be incorporated into the nascent virion during assembly and budding, and also implies some intracellular reservoir that retains UC781 following removal of the extracellular drug.
In agreement with this possibility, we found that brief exposure of uninfected lymphoblastoid cells to UC781 rendered these cells refractory to subsequent HIV-1 infection in the absence of extracellular drug (10). This UC781-mediated "chemical barrier" is very effective and rapidly established. As an example, 10 minutes incubation of uninfected MT-2 cells with 2 µM UC781 was sufficient to prevent subsequent infection of these cells by viral loads up to 2.5 x 105 TCID50. This UC781-induced chemical barrier to infection persists for prolonged times following exposure of the uninfected cells to the drug. Cells remained resistant to HIV infection for several days following removal of exogenous UC781. The apparent half-life of the UC781-induced chemical barrier in treated MT-2 cells was about 5.5 days (10), which corresponds to about five doublings of the cells.
The studies described above used lymphoblastoid cells. Sexual transmission of HIV may involve a variety of different cell types, including macrophages, epithelial cells, etc. We examined the ability of UC781 to establish a barrier to HIV infection of ME180 cervical epithelial cells (23). The epithelial cells were treated with UC781 or nevirapine, washed to remove all exogenous drug, and then exposed to HIV-1. Neither NNRTI was unable to prevent HIV-1 infection, as shown by the presence of integrated proviral DNA in the epithelial cells. The reasons for the inability of UC781 to protect epithelial cells from infection are presently unclear. However, this may be related to the mechanism of virus entry in these cells. As opposed to the usual attachment-fusion penetration of HIV-1 to CD4+ T cells, it has been shown that HIV-1 is taken into the cytoplasm of the ME180 cells via smooth vesicles in a process resembling non-specific phagocytosis (24). This difference in viral uptake may explain why UC781 does not protect these cells from HIV-1 infection. The amount of viral particles produced by the epithelial cells was also not altered by the pretreatment of the cells with UC781, as determined by the levels of HIV-1 p24 found in the supernatants. However, the infectivity of the nascent virus subsequently produced from the UC781-treated cells was attenuated in a dose-dependent manner and was virtually abolished at UC781 concentrations readily attainable in vivo (as determined by RT activity). In contrast, nevirapine was ineffective in this respect, further demonstrating that not all NNRTIs have microbicidal potential.
Preclinical studies of UC781 as an anti-HIV microbicide
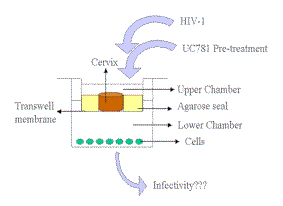 |
| Figure 4. Schematic diagram of the organ culture model used to study HIV-1 transmission through cervical tissue. Preparation of the cervical tissue for use in HIV studies has been detailed previously (25). Briefly, 5 mm circular pieces of ecto-cervical tissues, normal by pathological examination and collected from HIV-1 seronegative donors undergoing hysterectomy, were placed on the top chamber of a 12-well Transwell, with the epithelial layer oriented on top. UC781 was added to the media in the upper chamber and after 2, 5, 10 or 20 minutes the medium in the upper chamber was removed and the upper chamber was washed thoroughly five times with media. Fresh medium with virus was then added to the upper chamber, and incubation continued for a total of 5 to 6 days. Samples from the media in the lower chamber were withdrawn every few days and i) added to MT-2 or cMAGI cells for determination of viral infectivity, and ii) their p24 content was determined. As control for residual drug in the upper chamber, the last media wash was added to MT-2 cells and the capacity of HIV-1 to infect these cells was determined. |
| [View larger version of this image] |
The antiviral potency, stability, and safety of UC781 formulated in a lipophilic gel (ReplensTM gel) have been investigated (25). UC781 has no effect on the growth of microorganisms normally present in the vaginal flora and was stable (no loss of antiviral potency) following 4 hours of incubation at pH 3.5 (the estimated vaginal pH). UC781 was readily formulated at up to 5% in Replens gel and was stable in this form for at least 30 days at elevated temperatures (50°C). No local inflammation or damage to vaginal mucosal or epithelial layers was noted upon intravaginal administration of 5% UC781 in Replens to rabbits. Furthermore, preliminary assessment suggested that no systemic uptake of UC781 occurred following intravaginal topical administration of the UC781/Replens formulation.
It is estimated that the average human intravaginal dose of a microbicide gel would be about 1 ml. A microbicidal gel containing 5% UC781 would provide a UC781 concentration at least seven orders of magnitude higher than those needed to inhibit replication even of UC781-resistant HIV-1 and thus would likely provide an effective anti-HIV barrier.
UC781 and HIV infection in human cervical tissue explants
The data described above strongly suggest the potential of UC781 as an active component of an anti-HIV-1 microbicide. Recent studies concerning the ability of UC781 to inhibit HIV-1 infection in human cervical tissue provide additional support for this possibility. In these studies, we used a novel in vitro cervical tissue-based organ culture model, developed specifically to study the mechanisms of heterosexual transmission of HIV-1 (26). This organ culture model, illustrated in Figure 4, allows the maintenance of the natural architecture of the cervical tissue as evidenced by histology and quantitative immunostaining of cellular proteins. Both cell-free and cell-associated HIV-1 can be used to assess transmission of virus across the mucosal barrier.
UC781 appears to be relatively non-toxic to human cervical tissue, as no cell damage or decrease in cell viability was noted following overnight incubation of the cervical tissue with 20 µM UC781, as determined by histochemistry and MTT analysis (27). When cervical tissue was subjected to a short, 20-minute exposure to 1 µM UC781 contained in the upper chamber and then exposed to HIV-1 after thorough removal of the drug from the upper chamber, no infection of susceptible cells (MT-2, cMAGI, etc.) in the lower chamber was subsequently evident (Fig. 5). Infection of these cells was readily evident in control samples, that had not been treated with UC781.
In other experiments, pretreatment of the cervical tissues with 10 µM UC781 for as little as 5 minutes conferred total protection to the tissues from both M-tropic (Ba-L and ADA-M) and T-tropic (IIIB) laboratory strains, clinical virus isolates from different HIV-1 clades (B, C, G, and O), and from HIV-1 resistant to AZT or 3TC or both. A single exposure to UC781 provides extended protection from virus infection in the cervical tissue model, as infection was still prevented when the tissue was challenged with virus 48 hours after exposure to the drug.
Conclusions
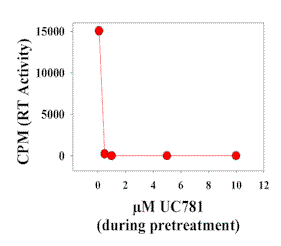 |
| Figure 5. Ability of UC781 treatment to prevent HIV-1 transmission in the cervical tissue model. Medium with and without UC781 was added to the upper chamber of Transwell plates containing cervical tissues from the same donor. After 20 minutes the upper chamber was thoroughly washed and media containing 5x104 TCID50 units of HIV-1IIIB were added to it. Five days later, medium from the lower chamber was tested for reverse transcriptase activity. |
| [View larger version of this image] |
The available data show that UC781 has anti-HIV-1 microbicide potential at several levels. First, UC781 inactivates cell-free virus. Second, UC781 attenuates the infectivity of nascent HIV-1 produced by infected cells after exposure to the drug, thereby inhibiting transmission of cell-associated virus. Third, UC781 is readily taken up and remains for extended periods within uninfected HIV-susceptible cells, thereby inhibiting infection during subsequent exposure to HIV. Furthermore, UC781 is very effective in preventing the transmission of HIV in a human cervical tissue explant model. These protective effects are established in minutes and persist for hours, important considerations in vaginal microbicides designed to prevent sexual transmission of the virus. Finally, UC781 is readily formulated into lipophilic gels that are already used for intravaginal application.
There have been some concerns about NNRTI-resistance issues in the use of NNRTIs such as UC781 in anti-HIV-1 topical microbicides. We consider these concerns unjustified. The use of NNRTI-containing microbicides is unlikely to result in the development of NNRTI-resistant virus, as microbicides are likely to be used in a prophylactic manner primarily by noninfected individuals, and there is little opportunity of HIV to remain under constant drug pressure in these cases. It is also unlikely that the use of NNRTI-containing microbicides will allow the transmission of NNRTI-resistant strains to naïve individuals. The concentration of UC781 readily attainable in lipophilic gel formulations is substantial, much higher than the EC95 for even the most resistant virus. In addition, as shown in rabbits (25), it is probable that no systemic uptake of UC781 will also occur in humans following intravaginal topical administration of the UC781/Replens formulation.
Topical anti-HIV microbicides are likely to comprise combinations of several active antiviral components. The microbicidal properties of UC781 are such that this compound should be given serious consideration for use in such formulations. Indeed, Biosyn, a Philadelphia-based company, has recently licensed the rights to develop UC781 as an anti-HIV topical microbicide. We look forward with great anticipation to the initiation of clinical trials in this respect.
References and notes
| 1. | UNAIDS, "Report on the global HIV/AIDS epidemic" (2000). Available online. |
| 2. | M. G. Fowler, S. L. Melnick, B. J. Mathieson, Obstet. Gynecol. Clin. North Am. 24, 705 (1997). PubMed. |
| 3. | J. Trussell, D. L. Warner, R. A. Hatcher, Fam. Plann. Perspect. 24, 20 (1992). PubMed. |
| 4. | D. Eich-Hochli, M. W. Niklowitz, M. Sieber, M. Opravil, R. Luthy, Int. J. STD AIDS 8, 69 (1997). PubMed. |
| 5. | T. Vos, AIDS Care 6, 193 (1994). PubMed. |
| 6. | B. Sondo, D. Sya, R. Pare, S. Kouanda, L. Savadogo, Sante 11, 111 (2001). PubMed. |
| 7. | R. Pauwels and E. De Clercq, J. Acquired Immun. Def. Syndromes Hum. Retrovirol. 11, 211 (1996). PubMed. |
| 8. | S. L. Rosenthal, S. S. Cohen, L. R. Stanberry, Sex Transm. Dis. 25, 368 (1998). PubMed. |
| 9. | S. McCormack, R. Hayes, C. J. Lacey, A. M. Johnson, Br. Med. J. 322, 410 (2001). PubMed. |
| 10. | G. Borkow, J. Barnard, T. M. Nguyen, A. Belmonte, M. A. Wainberg, M. A. Parniak, J. Virol. 71, 3023 (1997). PubMed. |
| 11. | M. A. Parniak, AIDS 15, S56 (2001). |
| 12. | J. P. Bader et al., Proc. Natl. Acad. Sci. U.S.A. 88, 6740 (1991). PubMed. |
| 13. | J. Balzarini, W. G. Brouwer, E. E. Felauer, E. De Clercq, A. Karlsson, Antiviral Res. 27, 219 (1995). PubMed. |
| 14. | J. Balzarini et al., Proc. Natl. Acad. Sci. U.S.A. 92, 5470 (1995). PubMed. |
| 15. | R. W. Buckheit Jr. et al., Antimicrob. Agents Chemother. 39, 2718 (1995). PubMed. |
| 16. | J. Balzarini, W. G. Brouwer, D. C. Dao, E. M. Osika, E. De Clercq, Antimicrob. Agents Chemother. 40, 1454 (1996). PubMed. |
| 17. | J. Balzarini et al., Mol. Pharmacol. 50, 394 (1996). PubMed. |
| 18. | J. B. McMahon et al., J. Pharmacol.Exp.Ther. 276, 298 (1996). PubMed. |
| 19. | R. W. Buckheit et al., Antimicrob. Agents Chemother. 41, 831 (1997). PubMed. |
| 20. | J. Barnard, G. Borkow, M. A. Parniak, Biochemistry 36, 7786 (1997). PubMed. |
| 21. | H. Zhang et al., AIDS Res. Hum. Retroviruses 9, 1287 (1993). PubMed. |
| 22. | H. Zhang, G. Dornadula, R. J. Pomerantz, J. Virol. 70, 2809 (1996). PubMed. |
| 23. | G. Borkow, H. Salomon, M. A. Wainberg, M. A. Parniak, submitted for publication. |
| 24. | X. Tan, R. Pearce-Pratt, D. M. Phillips. J. Virol. 67, 6447 (1993). PubMed. |
| 25. | J. Balzarini et al., AIDS 12, 1129 (1998). PubMed. |
| 26. | K. B. Collins, B. K. Patterson, G. J. Naus, D. V. Landers, P. Gupta, Nat. Med. 6, 475 (2000). PubMed. |
| 27. | P. Greenhead et al., J. Virol. 74, 5577 (2000). PubMed. |
| 28. | Research carried out by the authors was supported in part by grants from the Yael Research Fund (to G.B.) and from the American Foundation for AIDS Research and the Medical Research Council of Canada (to M.A.P.). |
| Copyright Information | Site map |